What Are Breast Implants?
Breast implants are medical devices that a plastic surgeon utilizes to enhance the size and shape of the natural breast. These structures vary by type and in the small details such as profile and general shape.
Dr. Donna Krummen dedicates time to discuss the details of each type of implant with each patient so that they are empowered to choose which best suits their desired outcome.

What Are Silicone Breast Implants?
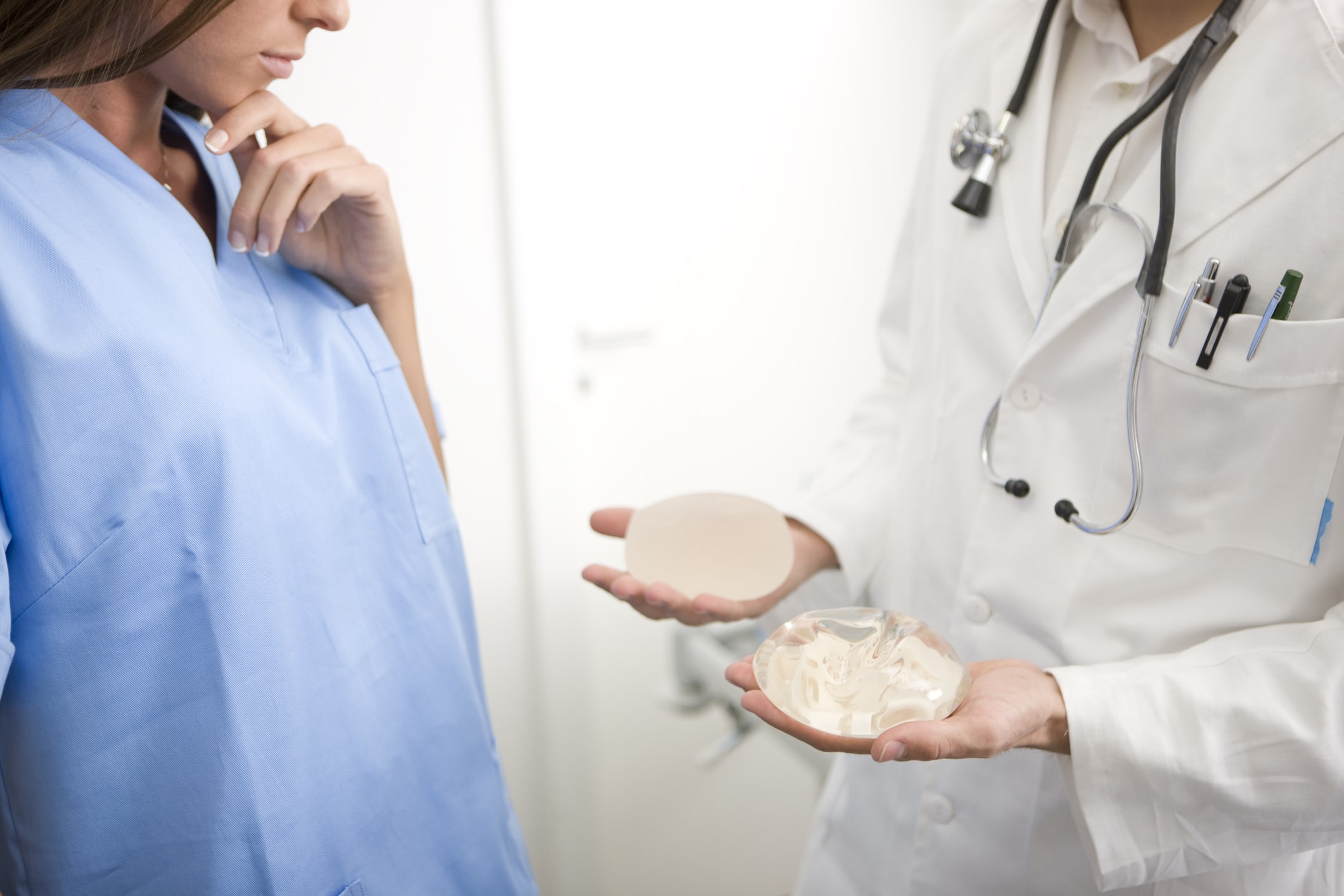
Silicone breast implants are medical devices that contain a thick silicone center within a silicone shell. This type of implant is pre-filled and therefore requires a slightly longer incision during surgery. Silicone implants are approved for women aged 22 and older for cosmetic purposes. Women of any age may receive silicone implants for breast reconstruction. Standard silicone implants are available only in round shapes but come in a wide variety of sizes.
What Are The Benefits?
The density and consistency of silicone are very similar to natural breast tissue. This results in an organic shape and feel, not one that is too firm to feel fully natural. Silicone implants are less likely to cause wrinkling or rippling in the skin, which makes them a good choice for breast reconstruction as well as for women who naturally have very little breast tissue.
What Are Saline Breast Implants?
Saline breast implants have an outer shell made of medical-grade silicone. This implant is inserted into the tissue envelope above or below the pectoral muscle in an unfilled state. Once in place, the implant is filled with sterile salt water to achieve the predetermined size discussed during the surgical consultation. Silicone implants are approved for women aged 18 and older.
What Are The Benefits?
A saline implant poses no risk of harm to surrounding tissues should a leak or rupture occur. Also, leak or rupture is more immediately known with silicone implants because the breast physically deflates as the saline gets absorbed by the body. Women who are interested in the smallest possible incision appreciate that saline implants are filled only once they have been situated beneath the breast tissue.
This type of implant, though typically less popular than silicone devices, provides more upper pole fullness than its alternatives. However, because saline is heavier than silicone, there is a bigger risk that this type of implant will “bottom out” due to gravity.

What Is The Difference Between Saline And Silicone Breast Implants?
The unique aspects of silicone vs saline and the lower risk of rippling make silicone breast implants a good choice for women with very little natural breast tissue. Saline implants, on the other hand, are an excellent option for women with sufficient fatty tissue on the breasts and the ability to have saline implants inserted beneath the muscle.
The differences in the consistency of saline versus silicone also affect the overall look and feel of the breasts after augmentation. Most women agree that silicone implants are softer and more like natural breast tissue than saline implants. However, when there is ample fatty tissue present, the feel of saline may remain quite soft.
What Are Gummy Bear Implants?
Gummy bear implants get their name from the popular chewy candy that holds the same shape all the way through. In medical terms, gummy bear implants are what we call form-stable silicone implants. These devices contain a thick silicone gel that is firmer than traditional silicone and therefore more likely to remain in place if the implant shell is broken. Gummy bear implants are available in round and anatomical shapes and a variety of sizes.
What Are The Benefits?
One of the primary advantages of gummy bear implants is the solidity of cross-linked silicone particles, creating form stability without diminishing natural feel and appearance. While some patients have reported that the firmness of these implants takes some getting used to, most agree that gummy bear implants provide a teardrop shape that mimics the all-natural breast.
How Do I Know Which Implant Is Best For Me?
The best way to determine which implant is best for you is to consult with an experienced, board-certified surgeon. Dr. Krummen can recommend options based on the extent of increase you desire, the natural shape of your breasts, the thickness of your skin, and various other aspects of your body frame and lifestyle. After receiving information from your plastic surgeon, you may want to take a few days or weeks to consider all of your options and how the predicted results of each would feel to “live in.”
How To Choose The Right Breast Implant Size
Dr. Krummen helps patients determine which size implant is best for them based on their overall shape and body frame. It may not be beneficial to come to your breast augmentation consultation with a particular cup size in mind.
Breast implants come in a variety of sizes, and also in varying profiles. The profile of a breast implant dictates how far the breasts will project after surgery. Therefore, profile and size are interrelated in achieving the outcome you desire.
What Are The Breast Implant Placement Options?
The two implant placement options are subglandular and submuscular.
Subglandular placement inserts implants under the breast glands but over the muscle. This situates the implants between the breast tissue and the muscle. This type of placement may be more comfortable in terms of recovery. However, subglandular implants are more easily seen and felt.
Submuscular placement inserts implants completely or partially under the chest muscle. The deeper placement may result in slightly more soreness or tenderness after surgery. However, implants are less visible and palpable when situated beneath both fatty and muscle tissue.
Are Breast Implants Safe?
Breast implants are FDA-approved medical devices that have been widely studied for more than twenty years. Research in the long-term safety of breast implants continues to be a priority for the FDA as well as organizations such as the American Society of Plastic Surgeons and the Plastic Surgery Foundation. Together, these organizations have formed the National Breast Implant Registry to collect information on breast implant procedures through which the quality of care for all patients can be improved.
How Can I Prepare for My Breast Implant Procedure?
Preparing for your surgery ahead of time can often result in an easier transition to recovery following your appointment. Many patients begin preparation for their breast implant procedure several weeks before their actual surgery by doing the following:
- Consulting your primary doctor about stopping medications. If you are taking medications, it’s essential to consult your doctor about the possibility of stopping them during your procedure and recovery to avoid complications.
- Implement a light exercise routine. Getting used to light exercise can help you avoid gaining weight while in recovery and can also help keep your body active in gentle ways.
- Eat healthy. Eating a balanced diet will also prevent weight gain and the alteration of the appearance of your breast implants.
- Invest in comfortable clothing, bedding etc. Ensuring that your come is comfortable during your recovery can help patients deal with the side effects of their surgery much easier.
How Long Will It Take to Recover from Breast Implants?
Recovery from breast implants can take, on average, about four to six weeks. Within the first two weeks of your recovery, you may feel soreness, tenderness, and swelling. These side effects are common and often subside after this time period passes. Prioritizing rest is important during recovery. It’s also often recommended to arrange for loved ones to help you navigate life at home while you heal.
During recovery, it is important to avoid high-intensity workouts and applying pressure to the chest area. Following your aftercare guidelines, as recommended by Dr. Krummen will ensure you avoid any complications during recovery. Soreness may continue for around two to three weeks, but after six weeks, most patients are able to return to work and their normal routines without any pain.
How Long Will The Results from My Breast Implants Last?
Breast implants are made to last at least ten years after your surgery, but oftentimes, they can last up to 20 years. With the proper care, many patients end up enjoying their implants for more than 20 years before needing a touch-up surgery. As your body naturally ages, over time, your implants will move and change with your body. In a couple of decades, many patients return to have their implants adjusted or refreshed after time has gone by.
Testimonials:
"Dr. Krummen, thank you so much for doing my breast surgery… You are a great surgeon, very caring. I would highly recommend you to anybody who would ask me for a suggestion. Thanks again for everything you have done for me."
"OUTSTANDING! Dr. Krummen and her staff are professional, friendly, knowledgeable, and helpful; their facility is extremely clean and efficiently run; Dr. Krummen is thoughtful, safe, and meticulous with patients in her care; I couldn’t be happier with my experiences there."
"Dr. Krummen and her staff are very friendly and accommodating. Dr. Krummen is patient and very approachable. She is easily available to answer questions. She is there for her patients! She responds promptly and courteous even after hours! You will feel comfortable working with her. My results are stunning! I couldn't be more pleased. She didn't try to over sell me, only recommended the procedure I needed to achieve my desired results. Rest assured knowing you are working with a professional! I will definitely reccomend her to all my friends and family."
Schedule A Consultation
To learn more about implants and the other breast augmentation services provided by Dr. Krummen, get in touch with our Cincinnati office by calling 513.985.0850 or by clicking here to submit an email contact form.